ISO 13485:2016
Medical Devices Quality Management Systems
SQNet Assessments, as an independent certification body, provides impartial ISO 13485:2016 audit and certification services, enabling organizations to demonstrate compliance, product safety, and consistent quality in medical device operations.
ISO 13485:2016 – Medical Devices Quality Management Systems
The medical device industry operates in a highly regulated environment where product quality, patient safety, and regulatory compliance are critical. ISO 13485:2016 is the internationally recognized standard that specifies requirements for a Quality Management System (QMS) specific to the design, development, production, installation, and servicing of medical devices.
Understanding ISO 13485:2016
ISO 13485:2016 applies to organizations involved in one or more stages of the medical device lifecycle, including manufacturers, suppliers, service providers, distributors, and organizations providing related services such as sterilization, calibration, or logistics.
The standard aligns with regulatory requirements across global markets and focuses on risk management, process control, and regulatory compliance rather than continual improvement alone.
Purpose of ISO 13485 Certification
Certification to ISO 13485:2016 demonstrates that an organization has implemented a QMS capable of consistently meeting customer and regulatory requirements for medical devices. It provides independent assurance that processes affecting device safety and performance are effectively controlled.
ISO 13485 certification is often a prerequisite for regulatory approvals and market access in many regions, supporting compliance with medical device regulations worldwide.
Apply for Certification
Connect with Our Certification Experts
Key Elements of a Medical Devices QMS
ISO 13485:2016 requires organizations to establish a QMS that includes the following key elements:
Quality policy and objectives
Risk management throughout the product lifecycle
Design and development controls
Supplier evaluation and control
Production and process validation
Control of monitoring and measuring equipment
Identification, traceability, and preservation of product
ISO 13485 Certification Process
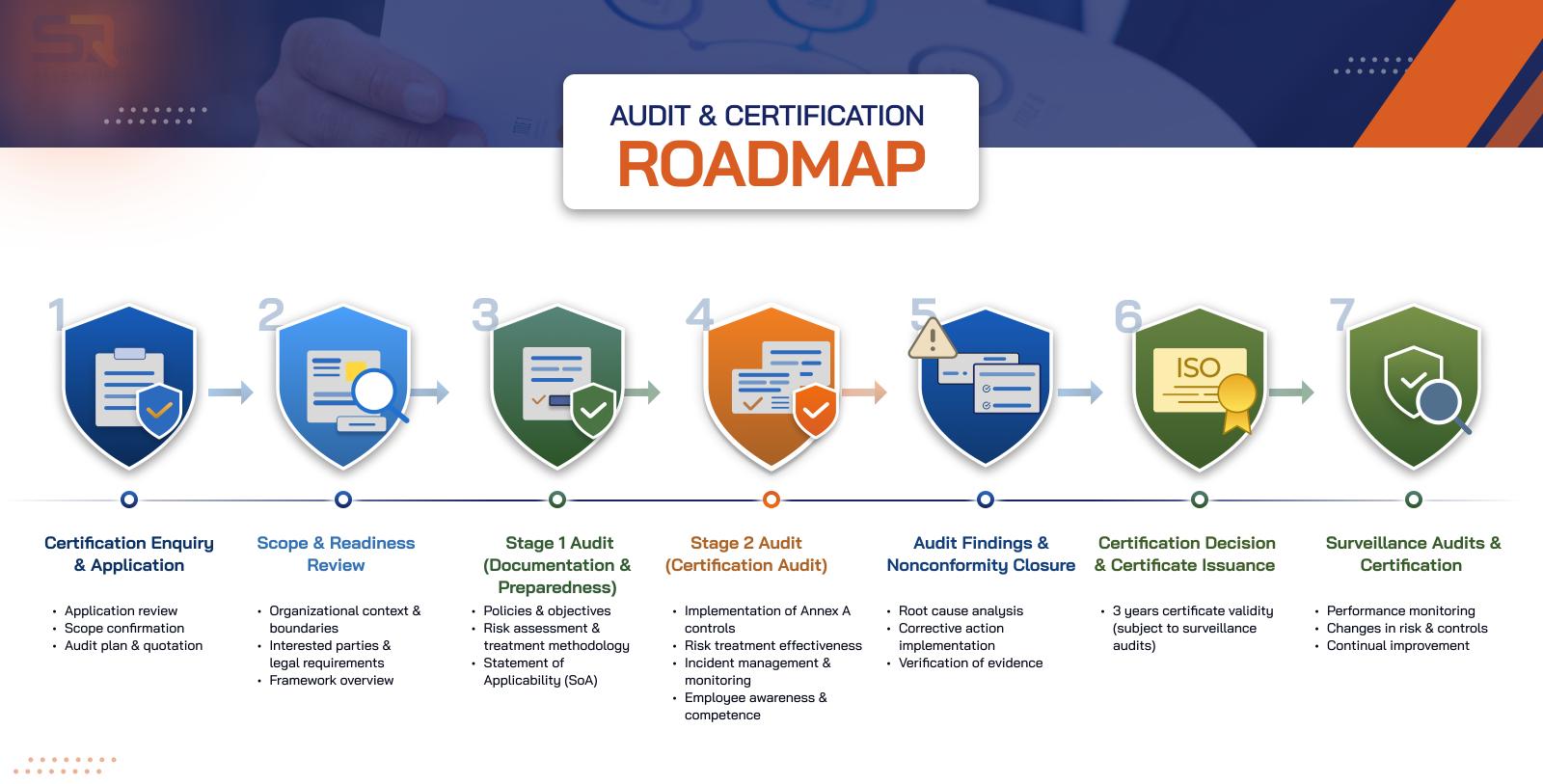
The ISO 13485 certification process conducted by SQNet Assessments follows internationally accepted certification practices and ensures impartial and objective evaluation.
Application & Scope Definition
The certification process begins with an application, during which the scope of the QMS is defined. This includes identification of medical device types, processes, and locations covered under certification.
Audit & Evaluation
Certification audits are conducted in two stages:
Stage 1 Audit – Reviews QMS documentation, regulatory readiness, and risk management processes
Stage 2 Audit – Evaluates effective implementation of QMS controls across operations
Audits assess compliance with ISO 13485 requirements, regulatory obligations, and effective risk control.
Certification Decision
Following successful audit completion and closure of any identified nonconformities, SQNet Assessments conducts an independent certification decision review before issuing the ISO 13485:2016 certificate.

Certification Validity & Surveillance Audits
ISO 13485:2016 certification is valid for three years, subject to annual surveillance audits. Surveillance audits ensure continued compliance, effectiveness of the QMS, and ongoing alignment with regulatory requirements.

Integration with Other Management Systems
ISO 13485:2016 can be integrated with other management system standards, such as:
ISO 9001 – Quality Management Systems
ISO 14971 – Medical Device Risk Management
ISO/IEC 27001 – Information Security Management
ISO 14001 – Environmental Management Systems
Integration supports consistent governance and efficient audits.

Commitment to Impartial Certification
SQNet Assessments is committed to delivering transparent, impartial, and credible ISO 13485:2016 certification services. Our structured audit methodology, competent auditors, and adherence to international certification principles ensure confidence in certification outcomes.

Key Benefits of ISO 13485
- Demonstrates compliance with medical device regulations
- Enhances patient safety and product quality
- Improves risk management and process control
- Strengthens market access and customer confidence
- Reduces regulatory and product liability risks
Key Changes in ISO 13485
- Alignment with the latest ISO management system structure
- Simplified and modernized Annex A controls
- Better integration with risk management and business objectives
- Enhanced focus on cloud security, threat intelligence, and data protection
Frequently Asked Questions
Certification is an independent verification process that confirms an organization’s management system, product, or service complies with applicable international standards. It enhances credibility, builds customer trust, and demonstrates commitment to quality, safety, and compliance.
Certification is applicable to organizations of all sizes and sectors, including manufacturing, service, IT, healthcare, construction, education, and public sector organizations, subject to the applicable standard and scope.
SQNet Assessments provides certification services for various international management system standards, including quality, environmental, occupational health & safety, information security, business continuity, and other applicable ISO and sector-specific standards.
The certification timeline depends on the organization’s size, scope, complexity, and readiness level. Typically, the process may take a few weeks to a few months from application to certificate issuance.
Most management system certifications are valid for three years, subject to successful completion of annual surveillance audits.
Stage 1 audit reviews documentation and readiness for certification.
Stage 2 audit evaluates effective implementation of the management system.
You can apply through the SQNet Assessments website or contact the team directly.